Vaccination of Indians Against COVID May Start in January’21: Health Minister Harsh Vardhan
By Nmami Life Editorial 27-Dec 2020 Reading Time: 5 Mins
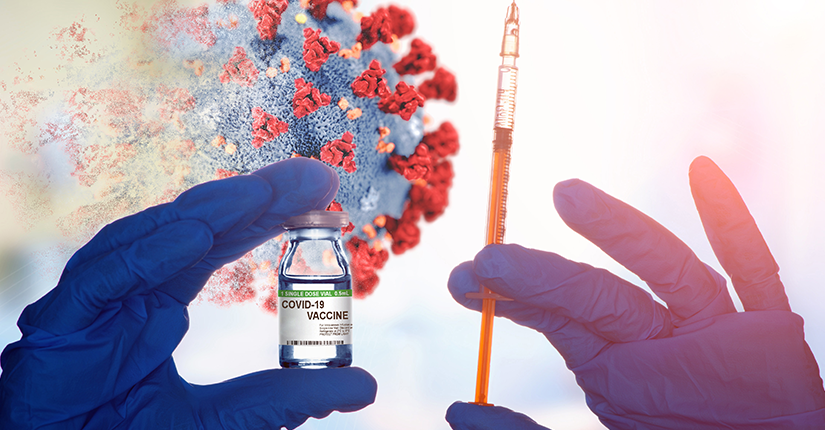
India is expected to begin it’s vaccination process against COVID-19 in January 2021. The government’s only priority has been vaccines efficacy and safety, says the Union Health Minister Harsh Vardhan.
“I believe that may be in January in any week or any stage, there can be a time when we may be in a position to permit the first COVID vaccine shot to citizens of India” Harsh Vardhan said in an interview.
He was also asked about India’s vaccination drive against coronavirus.
To which the minister said that the vaccines, including those who have applied for the emergency use, authorisation are being examined by the regulator.
India is nowhere less than any country when it comes to COVID-19 research and vaccines. Our utmost priority has been the efficacy and safety of the vaccine. We do not desire any compromise on that. Our regulators are sincerely analysing them,” the minister said.
Vardhan said on Saturday that the country’s health experts and scientists have been working on the development of an indigenous vaccine and in the upcoming 6 to 7 months India will have the capacity to inoculate about 30 crore citizens.
“Our health care experts and scientists have worked on the development of a vaccine by isolation and genome sequencing of the coronavirus and started an indigenous vaccine. In 7-6 months, we will have the capacity to inoculate about 30 crore citizens” he later said addressing the 22nd Group of Ministers meeting on COVID-19.
There are almost 6 vaccines for COVID-19 candidates in different clinical trial stages in the country including Sputnik V, Covishield, NVX-CoV2373, Covexin, and ZyKoV-D recombinant antigen-based protein vaccine. Apart from this, there are 3 COVID-19 vaccine candidates in the preclinical stage of which 1 of the candidates is in the pre-growth stage and is being researched by Aurobindo Pharma, Rajesh Bhushan the Union Health Secretary said.
“There are 6 vaccines which are in the clinical litigation stage. And 3 vaccines in the preclinical stage. Some of them can get licensed in the upcoming weeks but we cannot say anything at this moment of time because licensing is the domain of the national regulator, the Drugs Controller General of the country,” Rajesh said at a press conference.
Pointing out to Covexin, being manufactured by Bharat Biotech International Limited, Hyderabad in collaboration with the ICMR
(Indian Council of Medical Research) he said it is in the third phase trials and is on an inactivated virus policy. The manufacturers have appealed for the emergency use authorisation.
The United Kingdom became the first country worldwide to get authorised for the use of BioNTech and Pfizer for emergency reasons only earlier this month. The country is paving the way for the delivery of the vaccines.
Margaret Keenan was the first recipient of the vaccine from Northern Ireland. She is 90-year-old and was vaccinated at University Hospital in Coventry earlier on Tuesday.
Over to you:
There are 26,625 new COVID-19 cases recorded in the last 24 hours, India’s aggregative coronavirus cases reached 1,00,31,223, according to the MoHFW (Union Ministry of Health and Family Welfare) on Sunday.